If you are new to the life sciences sector, understanding the ins and outs of designing a manufacturing facility can be overwhelming. The nuances of life science buildings can be complex and foreign, even to the most seasoned project manager from a different industry. I learned a lot quickly when I changed industries, and I want to share my lessons with you.
Life sciences products go through various stages during the company’s “coming to market” process. Different regulations govern development and manufacturing facilities, so understanding which type of facility you need is the first step.
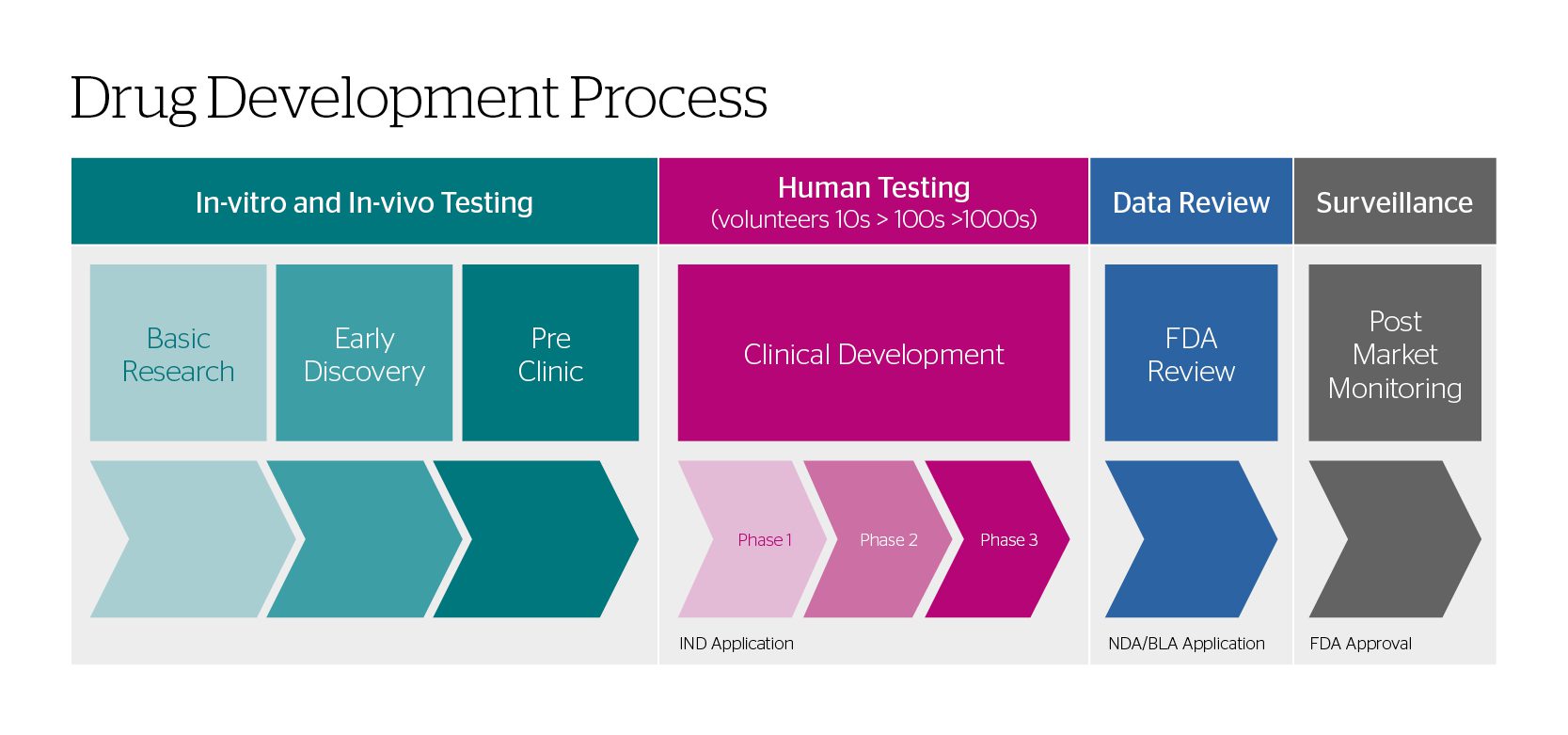
This blog looks at the first two steps in the drug development process and their respective general engineering standards: in-vitro/in-vivo testing and human testing.
The flow diagram below gives the typical steps in developing a new drug.
In-Vitro/In-Vivo Testing
When a pharmaceutical company is in the in-vitro/in-vivo testing stages of drug development, researchers find a point worth pursuing and begin pre-clinical research. The pre-clinical research typically starts with gathering insights into how a cell responds in a controlled, isolated environment (in-vitro) and then moves into studies within a living organism (in-vivo).
Generally speaking, these tests do not need to be conducted in cleanrooms with no contamination or infiltration. Preclinical laboratory studies must follow good laboratory practices (GLP) found in Title 21 of the US Code of Federal Regulations Part 58.1 (21 CFR Part 58.1). These regulations set minimums for conducting and reporting experiments, with little reference to the cleanliness of the space.
Human Testing
Once the results of the preclinical studies have determined dosing and toxicity levels, developers decide what to consider for each clinical phase, and begin the IND (Investigational New Drug) process.
The goal of the clinical development stage of the drug development process is
- Phase 1: identifying safety and dosage
- Phase 2: efficacy and side effects
- Phase 3: efficacy and monitoring of adverse reactions
Typically, facilities that conduct Phase 1 clinical trials are exempt from complying with 21 CFR Parts 210 and 211 unless the drug has been made available in a Phase 2 or Phase 3 study. If so, the Phase 1 facility must comply with 21 CFR 211. This regulation publishes the requirements of a cGMP (Current Good Manufacturing Practice) facility.
New terms to learn
You’ll also need to learn new terms very quickly. Don’t worry; you’ll hear them so often that they will become second nature in no time.
You’ll hear a lot about CMOs and CDMOS. The main difference between a CMO (Contract Manufacturing Organization) and a CDMO (Contract Development and Manufacturing Organization) is development. A CMO manufactures a pre-formulated drug, while a CDMO both develops and manufactures the drug.
A lab, or laboratory, is where researchers experiment with different solvents to create a therapeutic. These tend to be small rooms where prototypes are made before being sent off for government approvals. Specifications for each lab vary on a project-to-project basis.
A clean room is often bigger than a lab and is used to create bulk therapeutics after government approval is given. Generally, we refer to everything as a lab, but there are significant differences between a lab and a clean room. A particular difference is that a cleanroom is a room where therapeutics are created for human use – outside contaminants mustn’t get in, and that dangerous solvents do not get out. The allowable particulate count in the air defines the level of cleanliness of a clean room. We ensure that cleanrooms meet their requirements by using MAL (Material Air Lock) and PAL (Personnel Air Lock) and a specific number of Air Changes/per hour coupled with filtration. Anyone entering or exiting a cleanroom area must go through the PAL, while the equipment would pass through the MAL. Egress out of the clean room area is a big reason we separate the different airlocks.
A cleanroom can be used as a lab, but a lab is not usually used as a cleanroom.
The solvent storage area is critical to the safety of research and manufacturing facilities. It is a very specialized room – like a building inside a building – with walls that are independent of the rest of the outer structure. If there were a fire in the main building, the solvents stored in the solvent storage area would be safe. Vice-versa, the rest of the building will be protected if the solvents are toxic or flammable. .
The GMP (Good Manufacturing Practices) area is not just one room but a collection of rooms and contiguous square footage where people can travel safely without repeatedly moving through MALC and PALC rooms. PPE (Personal Protective Equipment) must only be worn when entering specific cleanrooms.
In future blogs, I’ll share more lessons I learned when I started my life sciences career. I learned so much about how the therapeutics that we use every day are developed and manufactured. It’s fascinating to see what’s entailed in creating a drug, garnering government approval, and exponentially increasing the output for a large market.